The electron is a fundamental particle having a mass of 9.11*10-31kg (energy equivalence of 0.511Mev) and a charge of 1.6*10-19c. It was discovered by J.J Thomson in 1897. In the standard model of physics, it is categorized as lepton under fermion. Electrons along with protons and neutrons form atoms. Electrons revolve around the nucleus in the atomic model. The mass of an electron (9.1*10-31kg) is 1/1836th of the mass of a proton.
So, in calculating the mass of an atom its mass is neglected. The deficit of electron in an atom produces +ve ion and excess of the electron produces -ve ion. The charge of the electron is considered as the elementary charge. Any charge is the integral multiple of charge of the electron. The flow of electrons produces electric current.
Characteristics:-
Electron is a fermion. It is a spin ½ particle and obeys Pauli’s exclusion principle i.e. no two electrons can have the same quantum state. Electron exhibits the properties of both waves and particles. According to de-Broglie, the wavelength of the associated wave with an electron is given by λ=h/p. When at rest it produces an electric field. But when in motion it produces a magnetic field.
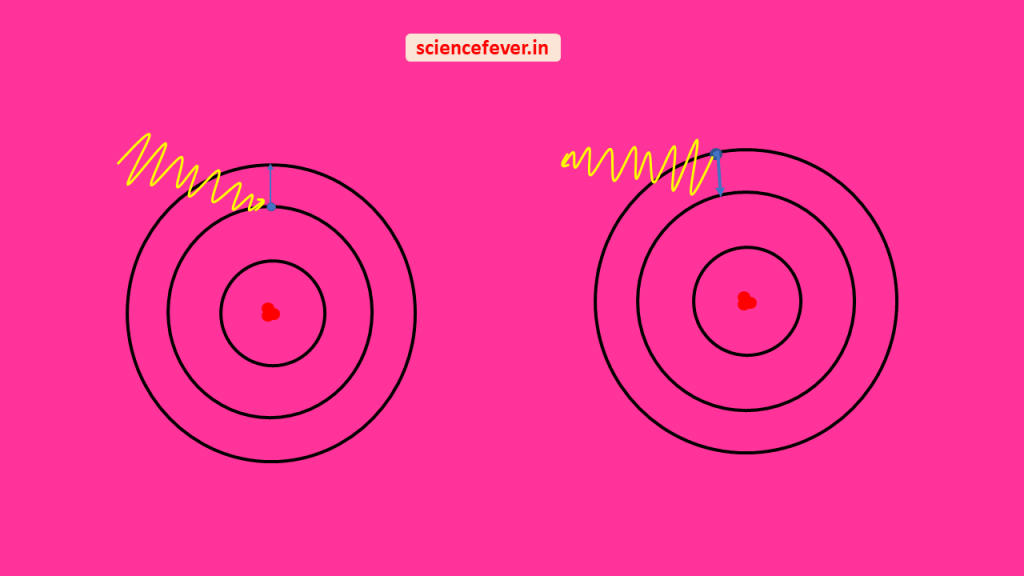
The force between two charged particles is governed by coulomb’s inverse square law. when an electron beam is passed through two narrow slits interference patterns can be observed. It also shows diffraction like the light. When an electron is exposed to an external electromagnetic field the force on the electron is called Lorentz force and given as.
\overset { \rightarrow }{ F } =q\overset { \rightarrow }{ E } +q(\overset { \rightarrow }{ v } \times \overset { \rightarrow }{ B } )Where,\\ \overset { \rightarrow }{ E } =Force due to the electric field
q(\overset { \rightarrow }{ v } \times \overset { \rightarrow }{ B } )=Force due to the magnetic field
The moving mass of electron is given by;
\\ m=\frac { { m }_{ 0 } }{ \sqrt { 1-\frac { { v }^{ 2 } }{ { c }^{ 2 } } } }Where, m0=rest mass
v=velocity of electron
c=velocity of light
When accelerating under force of nucleus it can absorb or emit quanta of energy to go to higher energy or lower energy state. It takes active part in the formation of bonds in chemistry. Two atom share electrons to form a bond.
Discovery of electron:-
1. In the year 1808 john Dalton said that matter is made of neutral atoms that cannot be made or destroyed.
2. Michael Faraday in 1803 found that when electricity is passed through electrolyte it conducts electricity and deposition of matter on electrodes took place. Which suggested electric nature of matter.
3. William crooks in 1879 discovered cathode rays through gases. This ray was travelling from cathode to anode.
4. J.J Thomson in 1897 placed the cathode ray tube between two oppositely charged plates.
5. He observed that the cathode ray is deflected towards the positively charged plate.
6. He also changed the materials of the electrode and gases inside the tube and he still found the same cathode rays. He confirmed that cathode rays consisted of negatively charged particles called “electron” and electrons are the fundamentals of atoms.
7. He deduced the ‘e/m’ ratio i.e charge to mass ratio as 1.758820×1011 C kg-1.
8. Its charge was calculated by Robert Millikan’s Oil Drop Experiment in 1909.
charge of electron, e=1.6\times { 10 }^{ -19 }C
\frac { e }{ m } =1.758820×{ 10 }^{ 11 }C.{ kg }^{ -1 } \\ \Rightarrow m=\frac { 1.6\times { 10 }^{ -19 }C }{ 1.758820×{ 10 }^{ 11 }C.{ kg }^{ -1 } } =9.104\times { 10 }^{ -34 }kg








Sometimes, we know the concept.. But we never try to understand how these concepts are made.. And how it relates another concept.. N this is the actual fact,we just go towards solving problems after knowing a little concept… But physics is all about concepts and honestly your articles helps us more than better gaining more knowledge… Keep it up we’ll always cheer you up…